From 2GreenEnergy Intern Fabio Porcu: Sodium Sulfur (NaS) Batteries
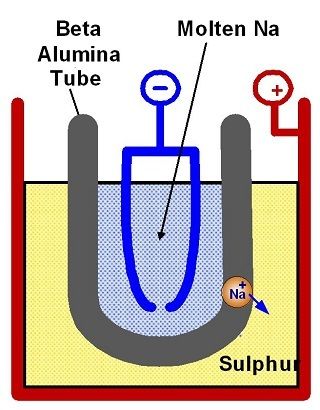
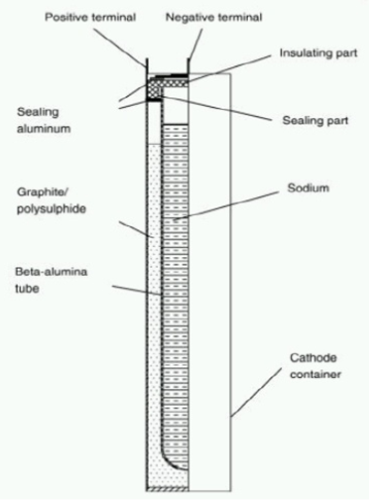
Essentially they consist of two basic materials: the anode is made of liquid sodium and the cathode is liquid sulfur. The two materials are separated by a solid electrolyte which is a beta-alumina; this is sintered, i.e., compacted and formed into a solid mass by heat and/or pressure, is made of material of ceramic type, and has the property to push through only the sodium ions. This enables the migration of sodium ions in the process of charge and discharge from side to side. The reactions that occur are the following:
ANODE 2Na →2Na+2e-
CATHODE XS+2e- →Sx(2e-)
GLOBAL REACTION 2Na+XS →Na2Sx
The sodium gives two electrons during the discharge phase, while the sulfur ions remain in close proximity to the electrolyte. The sodium migrates through the beta-alumina reaching the sodium and combines resulting Na_2 S_x. Here x is not defined because it passes by tetra sodium sulfide to sodium bisulphate. The increase in concentration, therefore the transition from the tetra, to the tri-sulfide, to bisulphate, gives us an indication of what should be the state of discharge. We must avoid producing sodium bisulphate; when it has a concentration of sodium bisulphate we reached the discharge limit of the system.
The NaS batteries have high energy density and power density, they have good stability at operating temperature, and offer low construction costs because they have sodium and sulfur as base material which are abundant and therefore low-cost.
The beta-alumina, on the contrary, is a material that requires a great deal of energy to produce; it’s expensive and very difficult to create. The structure of the beta-alumina determines the ability to get different performance from NaS batteries. If it is not handled well, it may explode: we must keep it at a temperature range between 270-350°C. It is important to maintain a constant temperature so the electrodes remain in a liquid state; if they become solids, the battery is no longer usable. For this reason internal devices are required to maintain a constant temperature.
Generally, during the charging process there is a production of thermal energy, enabling the battery to remain at the proper temperature. During the discharge phase the substances are cooled; thus, a part of the energy must be used to maintain the temperature. The management of the operation of charging and discharging is important because it enables the proper adjustment in the temperature.
These systems have a very high response speed; they react quickly to changes in load; they go from zero to the rated power in the order of milliseconds. They can also be overloaded up to 500% of their rated power: a battery of 50 V can be safely used at 250 V for as long as 15 minutes without developing any kind of problem, which is extremely interesting for stationary applications.
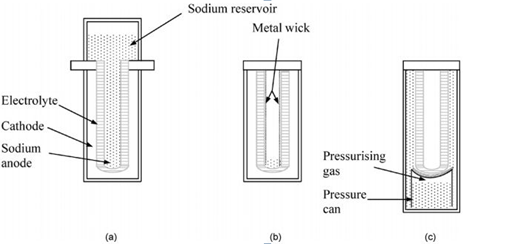
The beta-alumina (see photo) is similar to a pipe, like a melting pot, which precisely converts both the sodium and the sulfur. There are two configurations: one for high-power and one low. Low power means smaller dimensions of the beta-alumina, and smaller size of the cell.
NaS batteries are usually used in the electricity storage for grid support, in space applications, and in the field of transport for heavy machinery.

Possible error in article.
From the article:
“They can also be overloaded up to 500% of their rated power: a battery of 50 V can be safely used at 250 V for as long as 15 minutes without developing any kind of problem, which is extremely interesting for stationary applications.”
50 V and 250 V? Is it possible that 50 A and 250 A was intended?