Second Law of Thermodynamics
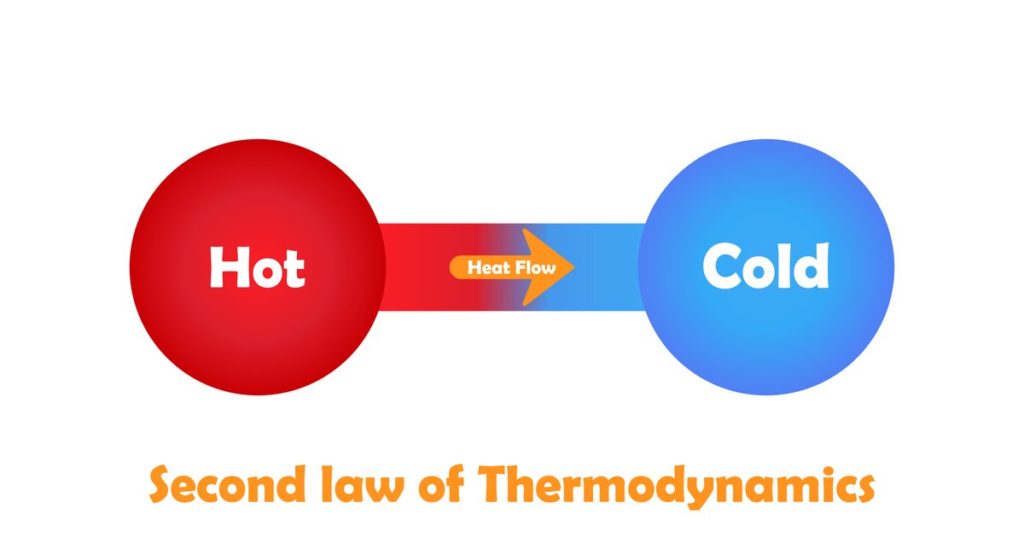
A corollary to this law is the principle that the amount of entropy (randomness/chaos) in a closed system is always rising. One might ask, as I did when I was young, Well, how is it that life on Earth is constantly evolving toward more order, rather than less? Is this evidence that God exists?
The answer I received is no, because this isn’t a closed system. Earth receives a huge amount of energy from the sun each day.
The second law of thermodynamics is the correct answer to the question posed by the high school physics teacher in the test shown below. I was amused by the kid’s response–and also the teacher’s; hope you are too.

